Glaucoma
Glaucoma Surgeon in Illinois & Iowa
What Is Glaucoma?
Think of your optic nerve as a cable connecting your eye to your brain—it carries all the visual information so you can see. Glaucoma slowly damages this nerve, cutting off the connection between your eye and brain.
Here’s what happens:
- Typically, fluid builds up in your eye, creating pressure
- This pressure damages your optic nerve over time
- You lose peripheral (side) vision first, but don’t notice it
- Without treatment, you eventually lose central vision, too
- Once vision is lost to glaucoma, it can never be restored
How Your Eyes’ Drainage System Works
In a healthy eye, fluid constantly flows in and out to nourish the eye and maintain its shape. Think of it like a sink with the faucet running and the drain open—everything stays balanced.
With glaucoma, the “drain” gets clogged or doesn’t work properly. Fluid builds up, pressure increases, and your optic nerve gets damaged.
Important note: Some people have high eye pressure but no nerve damage, while others have normal pressure but still develop glaucoma. That’s why comprehensive dilated eye exams are so important.
The Scary Truth About Glaucoma Symptoms
Are You at Risk?
Certain people have a higher risk of developing glaucoma:
Age and Family History:
- Over age 45 (risk increases with age)
- Family history of glaucoma (it can run in families)
Ethnicity:
- African Americans (6-8 times more likely)
- Hispanic Americans (higher risk, especially after age 60)
Medical Conditions:
- Diabetes
- High blood pressure
- Nearsightedness
- Previous eye injury
- Poor eye circulation
Medications:
- Long-term steroid use (including eye drops, pills, or injections)
Eye-Related Factors:
- Elevated eye pressure (but normal pressure doesn’t rule it out)
- Thin corneas
If you have any of these risk factors, regular eye exams are especially important!
How We Diagnose Glaucoma
The only way to detect glaucoma early is through comprehensive dilated eye exams that include:
- Eye pressure measurement (quick and painless)
- Optic nerve examination (requires dilated pupils)
- Visual field testing to check for blind spots
- Optic nerve imaging for detailed analysis
Annual eye exams are your best protection against vision loss from glaucoma.
Complete Glaucoma Discussion Series
Covers diagnosis, treatment, and answers to common questions
Advanced Glaucoma Treatments
Great news: Treatment options have come a long way! We can often control glaucoma and prevent further vision loss with:
1. Prescription Eye Drops
- Once-daily options available
- Lower eye pressure effectively
- The first line of treatment for most patients
2. Selective Laser Trabeculoplasty (SLT)
A revolutionary, painless laser treatment performed right in our office:
Benefits of SLT:
- Virtually no side effects, unlike some medications
- Reduces or eliminates the need for daily eye drops
- No scarring or eye damage like older laser treatments
- Effective for several years with just one treatment
- Painless procedure done in our office
Real patient success story: “After using drops for 2-3 years, my left eye grew worse. Dr. Wagle suggested the SLT laser. I was hesitant, but he explained there would be no pain. There was no pain, and my pressure went down a lot in my left eye. With these treatments, I know I can continue to enjoy my reading and get to my fitness center 3 days a week.” — Mrs. Segura
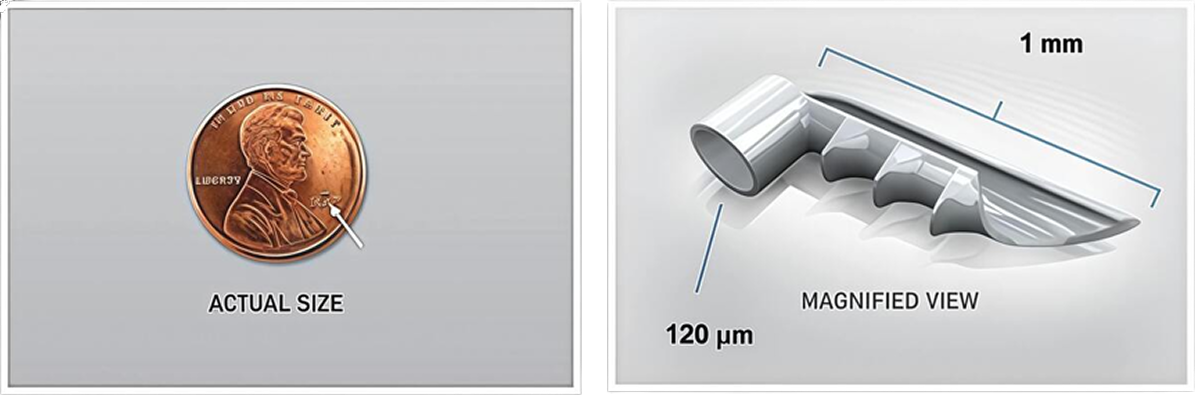
3. iStent® Micro-Bypass: The World's Tiniest Medical Device
If you need cataract surgery AND have glaucoma, we can treat both at the same time!
What is iStent?
- The world’s smallest medical device (20,000 times smaller than your cataract lens!)
- Works like heart stents to open blocked drainage
- Placed during cataract surgery
- Creates a permanent opening to improve fluid drainage
Benefits of iStent:
- May reduce the need for glaucoma eye drops
- Safely lowers eye pressure
Perfect for patients who:
- Have mild-to-moderate glaucoma
- Need cataract surgery
- Prefer fewer daily medications, reducing dependence on eye drops
Important: iStent is only used with cataract surgery and is suitable for specific types of glaucoma. We’ll determine if you’re a candidate during your evaluation.
See safety information below in Learn More About iStent Treatment.
Learn More About iStent Treatment
Indication for use: The iStent® Trabecular Micro-Bypass Stent (Models GTS100R and GTS100L) is indicated for use in conjunction with cataract surgery for the reduction of intraocular pressure (IOP) in adult patients with mild-to-moderate open-angle glaucoma currently treated with ocular hypotensive medication.
Contraindications: The iStent® is contraindicated in eyes with primary or secondary angle-closure glaucoma, including neovascular glaucoma, as well as in patients with retrobulbar tumor, thyroid eye disease, Sturge-Weber Syndrome, or any other type of condition that may cause elevated episcleral venous pressure.
Warnings: Gonioscopy should be performed prior to surgery to exclude PAS, rubeosis, and other angle abnormalities or conditions that would prohibit adequate visualization of the angle that could lead to improper placement of the stent and pose a hazard. The iStent® is MR-Conditional meaning that the device is safe for use in a specified MR environment under specified conditions, please see the label for details.
Precautions: The surgeon should monitor the patient postoperatively for proper maintenance of intraocular pressure. The safety and effectiveness of the iStent® have not been established as an alternative to the primary treatment of glaucoma with medications, in children, in eyes with significant prior trauma, chronic inflammation, or an abnormal anterior segment, in pseudophakic patients with glaucoma, in patients with pseudoexfoliative glaucoma, pigmentary, and uveitic glaucoma, in patients with unmedicated IOP less than 22 mmHg or greater than 36 mmHg after “washout” of medications, or in patients with prior glaucoma surgery of any type including argon laser trabeculoplasty, for implantation of more than a single stent, after complications during cataract surgery, and when implantation has been without concomitant cataract surgery with IOL implantation for visually significant cataract.
Adverse Events: The most common post-operative adverse events reported in the randomized pivotal trial included early post-operative corneal edema (8%), BCVA loss of = 1 line at or after the 3-month visit (7%), posterior capsular opacification (6%), stent obstruction (4%) early post-operative anterior chamber cells (3%), and early postoperative corneal abrasion (3%). Please refer to Directions for Use for additional adverse event information.
CAUTION: Federal law restricts this device to sale by, or on the order of, a physician. Please reference the Directions for Use labeling for a complete list of contraindications, warnings, precautions, and adverse events.
